Custom-Blended Additives Outperform Traditional Inoculants
For many decades ferrosilicon producers have sought to improve gray and ductile iron inoculants. This developmental work resulted from changes in the primary iron melting method. Medium-frequency coreless induction melting rapidly replaced cupola melting due to environmental requirements. More steel and purchased scrap were replacing some (or all) of the pig iron in the charges, and a general increase in the average melting temperature resulted in greater metal oxidation state. Further, cupola melted irons typically responded much better to inoculation than irons melted in coreless induction furnaces.
Ferrosilicon-based inoculants are made in large submerged arc furnaces using quartzite, coal, wood chips, and steel scrap. Mineral oxides rich in elements from Group IIA, IIIB, and IVB in the Periodic Table of Elements (such as strontium, barium, calcium, titanium, cerium, and magnesium) often are added to the smelting furnace or pouring ladle, to achieve the desired inoculant chemistry. However, there is a finite limit to the amount of these elements that can be added, or else the smelting and reduction reactions will be negatively impacted. Sulfur and oxygen are other important inoculating elements that cannot be added to the smelting furnace.
If the sulfur and oxygen content of the molten iron that is to be treated with an inoculant is insufficient, an abundance of carbides and chill may result. Thus, the only feasible way to ensure that the molten iron has sufficient levels of oxygen and sulfur is to mechanically blend sulfur and oxide-rich elements into the inoculant additive.
Group IIA, IIIB and IVB in the Periodic Table of Elements react with dissolved oxygen and sulfur to varying degrees to form atomic clusters of oxy-sulfide particles that have a crystalline structure similar to graphite. These surfaces greatly assist in graphite nucleation and prevent “undercooling” during solidification process. Undercooling can lead to carbides, poor graphite shape, low nodule quantity in ductile (S.G.) irons, and have an adverse effect on mechanical properties and machining characteristics.
In the last decade, to improve the performance of high potency rare earth based-inoculants, a thin coating of ferrous sulfide and ferrous oxide was applied as a surface treatment to the ferrosilicon-based inoculant. However, this approach provided only a limited number of oxy-sulfide particles that would adhere to the surface of individual inoculant particles. In addition, this “coating” was removed easily during shipping prior to being used. The only method by which suitable additions of oxygen and sulfur can be incorporated into any inoculating agent is by using suitable blending techniques and selecting proper particle sizing.
An example of adding controlled amounts sulfur with rare earths (cerium) to improve inoculation and reduce carbides was first demonstrated by R.L. Naro and J.F. Wallace in 1970 (see Figure 1.)
Figure 1: Effect of oxy-sulfide forming elements (Ce and S) on the chilling tendency of a4.3 carbon equivalent gray iron.
This research showed the importance of controlling both gray iron sulfur and rare earth levels in the molten iron. Balanced ratios of rare earths (cerium) and sulfur, without the presence of ferrosilicon drastically reduced undercooling, completely eliminated chill and promoted favorable graphite shapes in grey irons.
Using this concept, patented technology (U.S. Patent 6,293,988B) has been developed and is based on a ferrosilicon-free inoculant, mechanically blended alloy that contains high levels of calcium and stoichiometric amounts of sulfur and oxygen, similar to the Naro and Wallace research findings. Using proprietary blending techniques, this new alloy has demonstrated remarkable abilities to reduce shrinkage, improve inoculation (reduced chill, elimination of carbides) improve nodule count and nodule shape.
Alternate methods to introduce sulfur and oxides onto the surface of a cerium/calcium containing ferrosilicon based inoculant is limited to the amount of these materials than can be coated onto the surface of the ferrosilicon particle substrate, thereby limiting the critical amounts of sulfur and oxygen needed to boost inoculant potency. Late additions of sulfur and oxygen allow the other proprietary inoculating elements (calcium, aluminum, barium, et. al) to react in situ, and provide multiple times the nucleation sites of other, less potent inoculants.
Sphere-o-Dox (SOD) has shown remarkable abilities to solve many troublesome inoculation situations. SOD is a proprietary blend of oxy-sulfide forming elements that provide a high volume of graphite forming nuclei when added to molten gray or ductile irons. Not only has it replaced high-potency rare earth-containing inoculants at numerous foundries, but it can be used as an inoculant enhancer to improve the performance of all ferrosilicon-based inoculants, such as standard calcium-bearing, barium-bearing or rare-earth containing alloys. As a result, greater efficiencies during inoculation treatment have been obtained at significantly lower addition rates, in both grey iron and ductile irons, resulting in significant metal treatment cost savings.
An example of how SOD can be used as an inoculant enhancer is illustrated by the experience of Foundry A — a medium-sized producer of thin-section (0.25 in. or less), shell molded castings. For years, carbides have been a serious problem. Switching to inoculants and/or blend inoculants met with little to no improvement. The buyer of the castings had to anneal all castings to eliminate carbides. To reduce costs, the customer requested that the castings be produced as-cast to avoid heat treatment and improve machinability.
Foundry A produces ductile iron to a 65-45-12 ferritic specification in a 6,000-lb. medium-frequency induction furnace. A 1,200-lb. tundish ladle is used for magnesium treatment; 1.75% GloMag R6-8 magnesium ferrosilicon is added to a 1,000-lb. tundish ladle, and covered with 7 lbs. of cover steel. The standard post-inoculation practice consisted of adding 4.5 lbs. of Calsifer 75, a calcium bearing 75% ferrosilicon. A K15 cast in-mold inoculant insert was used in each shell mold. All attempts using numerous inoculant blends failed to eliminate carbides. The most successful combination of inoculants was found to be 3.0 lbs. of Calsifer 75, 3.5 lbs. of VP216, and 0.6 lb. of SOD added as a separate, independent addition to enhance the performance of the inoculants. The occurrence of carbides in thin sections was eliminated only when SOD was used.
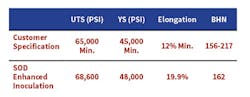
Table 1. Microstructure evaluation of the standard inoculation practice compared to using SOD-enhanced inoculation.
Microstructure evaluation of the standard inoculation practice compared to using SOD-enhanced inoculation showed nodule count increases of 20%, ferrite levels increased 190% and pearlite was reduced by 47%. The mechanical properties obtained from these same castings, is shown Table 1.
Both the microstructural and mechanical property improvements elicited favorable results from the end customer. In addition, eliminating the annealing cycle and improved machinability resulted in significant cost savings.
Sphere-o-Dox has been equally effective in casting pearlitic ductile iron grades, and it is just one example of how a custom-blended inoculant containing sulfur and oxygen can improve inoculation significantly.
Presently, many foundries throughout the world have adopted this non-ferrosilicon-based oxygen- and sulfur-containing inoculant to increase the potency of current inoculation practices, not only to reduce inoculant cost per ton but also to improve mechanical properties and machining properties.
Rod Naro is president and CEO, and David C. Williams is v.p. - Technology of ASI International Ltd. Visit www.asi-alloys.com