Double Checking for Changes in Binder Reactivity
Q: We run pH and Acid Demand Value (ADV) tests on our mold and core sands. Both of these tests measure the acidity or basicity of the sands. Why do we run both tests?
A: Both pH and ADV are related to the acidity and basicity of the sand. When we consider the most common type of foundry sand, that is silica sand, both tests are related to the impurities normally found in the sand.
Silica sand that is free of impurities will have a pH level of close to 7, which is, neutral on the acid and base pH scale. The ADV of this pure silica sand also will be zero. Impurities in the sand will change both the pH and ADV, depending on whether these impurities are water-soluble or -soluble in dilute acid. In order to understand the difference we must look more closely at each test.
The pH test for foundry sands uses 25.0 grams of sand mixed with 100 ml of distilled or deionized water adjusted to pH of 7. The sand and water is stirred for five minutes before the pH of the water is measured. The detailed method for determining pH of sand is found in The Mold and Core Test Handbook, 3rd Edition, AFS 5113-00-S. Because this method uses water, it is very sensitive to those impurities that are water soluble and can change the pH. Some examples of these types of impurities are sodium and potassium hydroxides, as well as some ammonia compounds. Even very small amounts of these water-soluble impurities can have a very large effect on the pH.
The ADV test for foundry sands uses 50.0 grams of sand mixed with 50 ml of distilled of deionized water adjusted to pH of 7, and 50 ml of dilute hydrochloric acid. The sand, water, and acid mixture is stirred for five minutes, then the mixture is analyzed to determine how much of the dilute acid has reacted with the impurities in the sand. A detailed method for the ADV of sand is found The Mold and Core Test Handbook, 3rd Edition, AFS 1114-00-S. The ADV number calculated in the test procedure is proportional to the amount of dilute hydrochloric acid that has reacted.
Very pure silica sand with no impurities will have an ADV number of 0.0. However, if the sand contains impurities that can react with the hydrochloric acid, the ADV number will increase. So, the more impurities in the sand that react with the hydrochloric acid and neutralize it, the higher will be the ADV number. Some impurities in sand will not react with the dilute hydrochloric acid and may themselves be acidic. In this case, the ADV number can be less than 0. This is the case with some furan no-bake reclaimed sands.
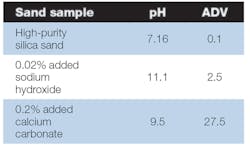
The main difference between the pH and ADV measurements are the nature of the impurities that are found in the sand. Water-soluble impurities will change the pH more than the ADV, and water-insoluble contaminants that react with acid may have a larger impact on the ADV. Sodium hydroxide is an example of a water-soluble material which has a marked effect on the pH. Calcium carbonate is a common contaminant in sand that is linked to limestone and sea shells. Calcium carbonate is rather insoluble in water, but it is soluble in hydrochloric acid and will react with it. Examining the effect of both pH and ADV on a sand contaminated with sodium hydroxide and the same sand contaminated with calcium carbonate demonstrates the difference between these two test methods.
Our experiment begins with a high-purity silica sand commonly used in foundry applications.When measured in our labs the pH of this sand was 7.16 and the ADV was 0.1. This sand was contaminated with 0.02% sodium hydroxide and 0.20% calcium carbonate. Table 1 shows the results of pH and ADV measurements on these artificially contaminated sands.While the pH and ADV both measure the acidity or basicity of the sand, the type of contaminant will influence one or the other more depending on the water solubility of the contaminant.
The change in the acidity and basicity of the sand can certainly impact the performance of the binder system you are using. Often the acidity and basicity of the sand can change the rate the binder system cures because the catalysts of many binder systems are either acids or bases. In some binder chemistries the pH will be more directly related to the rate the binder system cures or setting time. In other binder chemistries the ADV may be the best test to predict a change in setting time.
When you use and reclaim foundry sands the pH and ADV likely will change. First, the residual chemical binders, sand additives, mold adhesives, gating tile, riser sleeves, hot toppings, and even the refractory coatings that ultimately end up in the sand system can change the acidity and basicity of the reclaimed foundry sand. This is why experts recommend that you do not treat your sand reclaiming system like a “garbage disposal” by adding tramp materials, such as floor sweepings.
Additionally, if thermal reclaiming is used, the nature of these sand contaminants also can change. As an example, during thermal reclaiming the common sand contaminant calcium carbonate is decomposed and becomes calcium oxide. The calcium oxide reacts with water to form calcium hydroxide, which is much more water-soluble. The sample in Table 1 containing 0.2% calcium carbonate was baked for one hour at 1,000°C. We reran the pH on this sample and it increased to 11.8 while the ADV changed only slightly.
So, in summary, the pH and ADV of foundry sands both indicate acidity and basicity in different ways. Foundry sand pH and ADV can change as the sand is used and reclaimed. Both pH and ADV are useful lab tests to indicate changes in the sand and changes in binder reactivity you will experience.
Join the Conversation. Email Your Questions for ASK Chemicals
Share your insights, opinions, and elaborate on the questions and the experts' answer(s). You must be logged in to the website in order to post your comments.